- Research article
- Open access
- Published:
NaCl-related weathering of stone: the importance of kinetics and salt mixtures in environmental risk assessment
Heritage Science volume 9, Article number: 44 (2021)
Abstract
Salt weathering is one of the most important causes of deterioration in the built environment. Two crucial aspects need further investigation to understand the processes and find suitable measures: the impact of different climatic environments and the properties of salt mixture crystallization. We demonstrate the importance of kinetics in quantifying crystallization and dissolution cycles by combining droplet and capillary laboratory experiments with climate data analysis. The results proved that dissolution times for pure NaCl are typically slower than crystallization, while thermodynamic modelling showed a lower RHeq of NaCl (65.5%) in a salt mixture (commonly found in the built heritage) compared to its RHeq as a single salt (75.5%). Following the results, a minimum time of 30 min is considered for dissolution and the two main RHeq thresholds could be applied to climate data analysis. The predicted number of dissolution/crystallization cycles was significantly dependent on the measurement frequency (or equivalent averaging period) of the climatic data. An analysis of corresponding rural and urban climate demonstrated the impact of spatial phenomena (such as the urban heat island) on the predicted frequency cycles. The findings are fundamental to improve appropriate timescale windows that can be applied to climate data and to illustrate a methodology to quantify salt crystallization cycles in realistic environments as a risk assessment procedure. The results are the basis for future work to improve the accuracy of salt risk assessment by including the kinetics of salt mixtures.

Highlights
-
Salt weathering is one of the most important causes of deterioration in porous materials. The amount of salt crystallization cycles is influenced by different climatic environments and the properties of salt mixtures.
-
Sodium chloride in droplet and capillary experiments show a minimum time of 30 min for dissolution
-
Thermodynamic modeling shows a lower relative humidity equilibrium (RHeq) for sodium chloride in a salt mixture (commonly found in the built heritage) well below the RHeq of the single salt
-
The predicted number of dissolution/crystallization cycles is dependent on the measurement frequency (or equivalent averaging period) of climatic data
-
An analysis of rural and urban climate demonstrates the impact of spatial phenomena (such as the urban heat island) on the predicted frequency of salt cycles
Introduction
As a result of a UK-Belgium network project [1] and introduction to a joint PhD project [2] to tackle the challenges that face built heritage in a rapidly changing society and climate, salts and consequential weathering were considered understudied and vital to establish proper conservation management strategies. Salt crystallization-dissolution cycles have the potential to weaken and break down porous materials which ultimately leads to loss of the integrity, loss of function and value of cultural heritage. A wide range of literature is available considering the effects of salt crystallization in porous media, broadly defined as salt weathering with several important texts on the impact on natural stone materials such as the milestone references on natural landscape formation by Goudie and Viles [3] and the review by Evans [4]. In contrast, the literature about stone in the built environment has focused on practical approaches to addressing stone conservation (e.g., [5,6,7,8]). Fundamental questions, such as the mechanisms of crystallization and the development of crystallization pressure in porous media, remain open questions and active areas of research (e.g., [9,10,11,12,13,14,15,16,17,18,19]). Nevertheless, there is consensus that repeated cycles of crystallization and dissolution of hygroscopic salts is controlled by changing conditions in relative humidity (RH) and that repeated crystallization leads to the degradation of porous stone through the resulting weakening of intergranular bounds in the substrate.
Experimental testing and theoretical models are often used to predict the behavior of salt crystallization processes and develop sustainable risk assessment methodologies. Several experimental tests have been designed, debated and drafted in standard procedures, as is currently being done by the RILEM Technical Committee 271 ASC [19]. In theoretical models the critical relative humidity thresholds are determined as a method to predict if a single salt will undergo a phase change in certain environmental conditions, as described by e.g. Sabbioni et al. [20]. However, accurate thermodynamic calculations are based on ionic activities, which can introduce error as kinetics are not considered. Additionally, the deliquescence points of salt mixtures cannot be directly determined from those of the single salt components in the mixture, so they must be calculated, as described by Steiger et al. [21]. To determine the critical crystallization points for salt mixtures the ECOS/RUNSALT model [22, 23] is the only available model capable of predicting the behavior of salt mixtures found in the built environment under changing climate conditions. The system is based on the thermodynamic approach of Pitzer (based on mole-fractions rather than molalities) and represents a major achievement, enabling the prediction of the equilibrium behavior of salt mixtures. However, the kinetics of salts are not considered, and the model has several limitations. The constraints in which the results of such a model should be interpreted are rarely published [24]. Furthermore, fundamental research on salt mixtures can only be found in recent literature and is rare, e.g. [25,26,27,28].
The dependency of salt phase transitions on temperature and relative humidity, illustrated by the ECOS/RUNSALT outputs, underpin climatic control to mitigate salt damage. Therefore, this environmental risk has frequently been assessed by testing meteorological observations to parameterized relevant phase transitions. Grossi et al. [29] and Sabbioni et al. [20] have used thresholds of 75.3% RH and 75.5% respectively, as indicators for sodium chloride (NaCl) crystallization-dissolution cycles, testing average daily relative humidity from meteorological observations and taking negative crossings (crystallization) as a proxy for crystallization-dissolution cycles. Establishing a correlation between daily observations and monthly averages for a certain climate allowed them to link the environmental risk to different climate and climate projections. Benavente et al. [30] used a similar single threshold approach of daily averages for NaCl phase transitions for a case study on the Postumius Tomb (Spain), but considered a critical relative humidity difference of 10% for NaCl, setting the RH threshold at 65.3% for two consecutive days. This single-threshold approach can be justified as the relative humidity equilibrium (RHeq) of non-hydrating sodium chloride (NaCl) has a low temperature-dependent variation. Both Grossi et al. [29] and Benavente et al. [30] have used a more complex, temperature-dependent indicator for the hydrating sodium sulfates. Godts et al. [24] counted phase transitions of complex salt mixtures based on thresholds of the ECOS/RUNSALT outputs for a fixed temperature, using six- and twelve-hourly RH variations in the case of the St. James Church (Liège, Belgium). Menéndez [31, 32] has considered relative humidity changes and temperature to estimate the occurrence of salt transitions.
In response, this paper presents the results of an investigation on salt weathering assessment methodology that considers the temporal and kinetic behavior of salts present in porous media, in urban or rural environments. To illustrate the methodology, we consider an ideal situation with pure unconfined and confined NaCl directly influenced by changing RH and NaCl in a mixture. The dissolution and crystallization rates of NaCl under realistic changing climatic conditions are investigated. This in turn is the basis for continuing research [2] to identify phase transitions of salts in a mixture and predict the damage potential in changing climatic conditions, past and future. The aim of this paper is to identify the limitations of current approaches, the challenges to better risk assessment, and the future work required to enable them or address these (e.g. laboratory work). Specifically, this paper wants to guide future development of sustainable conservation and risk management strategies in order to mitigate the harmful impacts of salts.
Experimental methodology
Dissolution and crystallization properties of NaCl—droplet experiment
For the purpose of this methodological study, the dissolution and crystallization times of a NaCl crystal/solution are considered in droplets (Fig. 1). The obtained values are determined via time-lapse videos (5 s. resolution) (3D-digital microscopy, HIROX—imaged at a magnification of 160) by recording the solution/crystal under changing RH in a microclimate chamber (GenRH/Mcell, N2 gas flow: 200 sccm: standard cubic centimeters per minute). A NaCl crystal (80 ± 10 μg) (EMSURE® Merck, NaCl for analysis 1.06404.0500) is placed in the chamber (Mcell) on a carbon adhesive tape.
Example images of the droplet experiment a NaCl solution at 90% (start RH), b onset of crystallization (exaggerated to visualize the crystal—orange arrow) and c completed crystallization of NaCl at 70% (target RH). Imaged at a magnification of 160 with digital microscopy (HIROX) in a microclimate chamber (GenRH/Mcell)
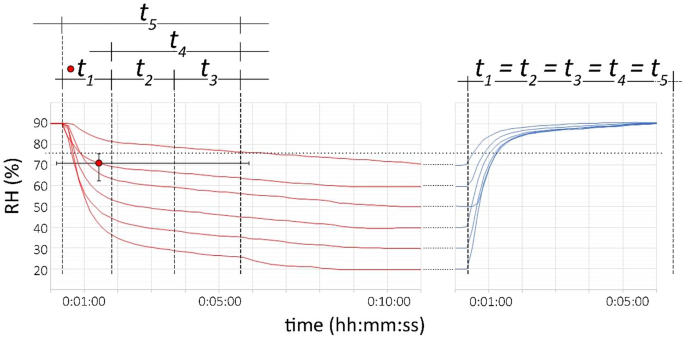
Twelve steps of RH changes are carried out at a temperature of 20 ± 1 °C. Each step considers a start and a target RH. The dissolved crystal (diluted salt solution) is conditioned at 90% RH (Fig. 1a, respective concentration: 2.85 mol kg−1 and droplet diameter: 409 μm), followed by a decrease to a lower target RH (respectively, 70%, 60%, 50%, 40%; 30%, 20%). When complete crystallization is noticed, the RH is increased back to 90% until complete dissolution. Specifically, the experiments have subsequently been carried out under the following RH cycles: 90%-X%-90%; with X = 70%, 60%, 50%, 40%; 30%, 20%. The effective crystallization time starts at first microscopic observation of a crystal (Fig. 1b), that is when the crystals appear to grow, and ending when growth is no longer visible, thus when the supersaturated solution film at the interface crystal/surface is no longer observed under the microscope (Fig. 1c). For the dissolution time we consider the moment when wetting of the crystal becomes visible, followed by complete dissolution of the crystals.
The target RH in the equipment (crystallization step) is reached between approximately 8 to 11 min, respectively decreasing time with increasing RH steps, while the opposite occurs for the return to start RH (dissolution step) the time for the equipment to reach the targets is approximately 3 and 6 min, respectively increasing time with increasing RH steps (Fig. 2). This paper describes different time steps considering:
-
t1: the time the equipment needs to reach below the RHeq (75.3% at 20 °C).
-
t2: the time when the equipment reaches the RHeq until the first visible crystal or surface wetting, which can be considered as the induction time.
-
t3: the time for the crystals or solution to fully grow or dissolve, thus the effective growth/dissolution times (not to be confused with growth rate). t3 = t5 − t4
-
t4 = t2 + t3 (the total time when the equipment reaches the RHeq until the end of visible growth/dissolution).
-
t5 = t1 + t2 + t3 (the total experimental time).
RH gradients for the crystallization and dissolution experiments (respectively red and blue curves). The horizontal dotted line at 75.34% indicates the RHeq of NaCl at 20 °C. The average time (first recorded datapoint) for the equipment to reach below the RHeq is shown as the red dot (including error bars). The horizontal error bar shows how the equipment needs more time to reach the RHeq during the crystallization experiments, with 0:05:53 for the experiment form 90 to 70% RH and less than a minute for the following experiments. The considered interval times (from t1 to t5) are shown as an example and explained in text above
For the dissolution experiments the deliquescence (first observed liquid film on the crystals) is observed immediately when the RH target is set to 90%. The flow of water molecules, at a rate of 200 sccm, is faster than the RH probe can measure, thus t1 equals t2, t3, t4 and t5 as the effective dissolution time.
Dissolution and crystallization properties of NaCl—capillary experiment
Additionally, the dissolution and crystallization times of NaCl were investigated in round glass capillaries (inner diameter: 100 µm). A NaCl solution (4 mol kg−1) is introduced into the capillaries in varying volumes and depths (see results) and is left to crystallize in four separate capillaries under lab conditions (Fig. 3a) (approx. 60% RH and ≈ 21 ± 1 °C). Four capillaries are chosen to represent the influence of different volumes and depths. Afterwards the samples were conditioned at 90% RH and 20 °C until the solution stops expanding (Fig. 3b), which is when the solution concentration reaches 2.85 mol kg−1 (the equilibrium concentration at 90% RH). The dissolution and crystallization times of NaCl were investigated with the same method as described above for the droplet, but limited to one RH cycle: from 90 to 20% back to 90% RH. The duration for the equipment to reach the target RH was (as explained above) 00:08:25 and 00:05:44 for respectively the crystallization (90 to 20% RH) (Fig. 3c) and dissolution (20 to 90% RH) experiments.
Example images of the capillary experiments a the position of the crystals before the experiments, b NaCl solution at 90% (start RH) and c completed crystallization of NaCl at 20% (target RH). Note the location of the crystals deeper into the capillary compared to the image on the left. Visualized at a magnification of 160 with digital microscopy (HIROX) in a microclimate chamber (GenRH/Mcell)
The total length of the solution and distance from the capillary entrance to the solution (inner sides of meniscus) is measured before lowering the RH. Both measurements are taken to respectively calculate the solution volume and consider the influence of the depth of the solution on the crystallization time. The volume of the solution in the capillary is calculated based on equations. 5 and 6 in Shen et al. [25]. Unlike Shen et al. we do not present results on the supersaturation at the onset of crystallization, but the total time of completed crystallization/dissolution with respect to the distance from the capillary entrance of the solution in the capillary.
NaCl in a salt mixture—thermodynamic modeling with ECOS/RUNSALT
To illustrate the effect of the presence of other salts on the RHeq of NaCl, the crystallization behavior of the single salt and a realistic complex mixture were modeled using ECOS/RUNSALT freeware [22, 23]. The mixture represents a commonly found solution in deteriorating building materials in Belgium. Ion chromatography results derived from a drill sample taken at a height of 25 cm and to a depth of 2 cm from a deteriorating limestone (in the Cathedral of Antwerp, Belgium) are used as input for the model. Specific information concerning the limitations and input method of the model are described in Godts et al. [24]. When using the model, equimolar contents of calcium and sulfate ions, considered as the gypsum content, are excluded. Also, an access of calcium ions is excluded which is associated with a small amount of calcium carbonates dissolved during the extraction process in ultra-pure water. The balanced input data for the ECOS/RUNSALT model is:
Cl− | NO3− | SO42− | Na+ | K+ | Ca2+ | Mg2+ | |
|---|---|---|---|---|---|---|---|
mol kg−1 | 0.072371 | 0.139891 | 0.022594 | 0.213874 | 0.043576 | 0.000000 | 0.000000 |
Predicting the occurrence of dissolution cycles—meteorological data resolution (spatial and temporal)
To study the impact of data properties when using climate data to estimate the occurrence and risk of salt crystallization cycles, a threshold-based indicator is applied to two datasets with high time resolution, representing urban and rural climates from July 2016 to June 2017. The urban and rural climates are represented by measurements at two locations. A typical ‘urban’ climate is represented by climate monitoring in central Ghent, a mid-sized city in the west of Flanders, Belgium. The corresponding ‘rural’ climate is represented by the climate monitored in Melle, a region several miles outside of Ghent without significant anthropogenic impacts on the local environment. The data was originally collected as part of a project to produce high-quality climate monitoring of several areas within the urban area of Ghent [33]. The local environment is monitored at a 1-min frequency.
The indicator represents the predicted number of instances in which dissolution would likely occur by comparing average RH% between subsequent periods of time. To evaluate the importance of data resolution on the estimated number of dissolution/crystallization cycles, an ‘averaging window’ of several difference widths, ranging from 1 min (the data frequency) to 24 h (used previously in humidity fluctuation calculations by Arfvidsson [34] was considered. The averaging window is an analogue for climate data recorded at wider time intervals, although these can be recorded as either individual measurements taken at regular intervals or the average of several measurements taken within that time period. Similar events concerning salt crystallization can be missed if the resolution of the data is too coarse or certain environmental factors are not considered such as rainfall, wind velocity, surface condensation and direct sunshine. For example, when dissolution happens after rainfall, the solution might be undersaturated and the necessary time before supersaturation is reached is likely to take more time. Even though the influence of above mentioned environmental factors are not taken into consideration in this study, it is likely that the crystallization time will remain significantly below the dissolution time, also for undersaturated solutions.
Two thresholds are considered in this paper:
-
75.5%, representing NaCl present as a single salt
-
65.5%, representing the behavior of a salt mixture that includes NaCl
Previous work has used a single threshold of 75.3% to study phase transitions [29]. However, herein a threshold of 75.5% is considered, based on the water activity (aw) of NaCl determined for a range of ambient temperatures. Although the temperature in both locations varies over the course of the year, the mean temperatures at the urban and rural sites were 12.5 °C and 11.5 °C, respectively. Based on the precision of the measured temperature data, these correspond most closely with aw = 0.755 (at 10 °C for a saturated sodium chloride solution 6.135 mol kg− 1). It also remains important to keep in mind that crystallization is affected by a range of different factors (see [18]) and dissolution of salt crystals takes more time compared to crystallization. Furthermore, a threshold of 65.5% RH is considered as the RHeq of NaCl tends to shift in a mixture with other ions, thus representing a more realistic situation.
Results and discussion
Dissolution and crystallization times of NaCl—droplet experiment
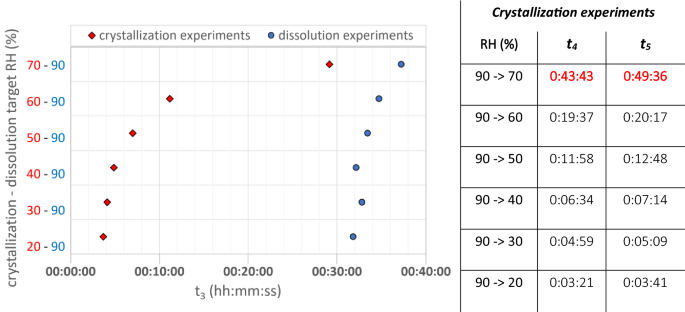
The dissolution and crystallization times of an unconfined NaCl droplet are illustrated in Fig. 4. The effective growth time (t3) of NaCl crystallization is faster compared to dissolution for all the experimental targets and comparable time steps (t4 and t5), except for the target from 90 to 70% RH. In this case t4 and t5 take respectively 0:06:39 and 0:12:32 longer compared to the dissolution time where t3 = t5 (as explained in the methodology). A correlation is found between RH ranges and dissolution/crystallization times. The wider the RH gap the faster the phase transition occurs. However, crystallization and dissolution do not have the same behavior, both rates increase when the RH range increases, but not in the same proportion. Crystallization times follow a power approximation while dissolution follow a more linear behavior.
Effective crystallization (growth) and dissolution times (t3) of unconfined NaCl crystal/droplet, determined by (GenRH/MCell; Hirox) at different RH targets between (20 and 90%). For the dissolution experiments t3 = t1 = t2 = t4 = t5, while for the crystallization experiments t1 and t2 can be derived from the data in the image and table to the right, with t1 = t4 − t5 and t2 = t4 − t3. Times t4 and t5 represented in red illustrate the times when crystallization takes longer compared to dissolution
Similar results were found in Desarnaud et al. [35]. They studied the nucleation and dissolution rates of NaCl crystals from 0.1 µl droplets of a saturated NaCl-solution (6.16 mol kg−1). The phase transition of NaCl crystals were observed from 50 to 100% and vice versa at a controlled temperature of 21 ± 1 °C. They observed that, at 50% RH the total time of NaCl-crystallization in a droplet of 0.1 µl on a hydrophilic surface is 5 min (± 0.1), while the total deliquescence time for the crystallized salt (0.36 mg) at 100% RH is two times longer (10 ± 2 min).
Dissolution and crystallization times of NaCl—capillary experiment
The effective crystallization and dissolution times (t3) of confined NaCl solution/crystals at respectively 20% and 90% RH (≈ 21 ± 1 °C) are presented in Table 1 (t3 C and t3 D). At the onset of the experiments, when dissolution is initiated, the crystals are closer to the entrance of the capillaries (d1) compared to distance of the solution meniscus to the entrance after completed dissolution (at 90% RH) (d2). To clarify, the experiments starts with the crystals in capillary 2 located directly at the entrance, thus 0 μm (d1). The crystals are also partly positioned on the outer edge of the capillary, subsequently during dissolution the crystals dissolve and the solution is pulled inside due to capillary forces (d2). Afterwards when crystallization is completed the crystals are located further into the capillary. Logically the amount of salt that needs to dissolve and the available surface area of the crystals will influence the dissolution time.
The above influencing factors are clearly illustrated as a correlation is distinguished between the distance of the crystals/solution to the entrance of the capillaries and the effective time (t3) for the crystals to completely dissolve. For example, this time is approximately 22 min for capillaries 1 and 2 where the crystals are near to the entrance. While for capillaries 3 and 4 where the crystals are significantly further away from the entrance the time increases to 68 and 144 min respectively (t3 D). This is further confirmed by the smaller solution volume (V1) in capillary 3 compared to 2 at the end of dissolution and solution expansion. In contrast, at completed dissolution the volume of the solution continues to expand until it reaches equilibrium at 90% RH, this process is less dependent on the solution volume or depth in the capillary with an added time after t3 of approximately 70 ± 6 min on average for the four solutions.
Just as in the dissolution experiment each capillary contains a different volume of solution at the start of the crystallization experiment. The start volumes and distance of the solutions in the capillaries at 90% RH are shown in Table 1 (V1 and d2), at this point the solutions reached a concentration of 2.85 mol kg−1. When the equipment is set to 20% RH, the RHeq is reached after 50 s (t1), while after approximately 4, 6, 8 and 16 min (t2) crystallization starts. Just before crystallization the solutions volumes (V2) decreased to a minimum (reaching supersaturation). The distance (d3) of the solutions from the entrance of the capillary increased compared to (d2). The effective crystallization times (t3 C) are significantly below the dissolution times (t3 D). The complete experimental times for the crystallization are respectively 8, 13, 12 and 34 ± 0.5 min (t5). The combined experimental times for the crystallization experiments remain far below the dissolution times.
A solution with less volume deeper in the capillary can crystallize at the same rate as solutions with more volume near the surface, however dissolution takes longer at larger depths into the capillary and is less dependent on the volume of the solution. In Desarnaud et al. [36] the same dependence of the distance of the solution from the capillary entrance has been found especially for the induction time (t2). The complete dissolution time of a NaCl hopper crystal of 2.31 ± 0.1 mg is 34 ± 5 min, while the total crystallization time of the same amount of NaCl was twice as fast, with 17 ± 3 min, yet two times slower compared to droplet experiments. The results confirm the importance of focusing on dissolution rates rather than faster crystallization rates when counting crystallization/dissolution cycles in realistic environments.
The approximation of crystallization cycles described further is significantly influenced by the location and the volume of the salt solution, comparable to salts either at the surface of a porous material or within the pores. Since dissolution takes more time, the approximation of the number of possible cycles (phase transitions) that can occur in a given environment should be based on the minimum dissolution times. Additionally, there are a wide range of factors that will further influence the results in realistic situations on site, such as:
-
Pore- sizes, structure, filling (crystals/solution), impurities (pollution, dust, organisms, etc.)
-
Depth (location) of the salt (solution) to the surface
-
Orientation, water load (rain, rising damp, infiltrations, etc.), wind and sun exposure, and their respective rates of change
-
Boundary conditions (building structure, materials, interfaces, past treatments, biological contamination, etc.)
The above experiments characterize an ideal situation with pure unconfined and confined NaCl directly influenced by changing RH. Representing respectively a single salt at the surface of a stone and just below the surface in the first pores. However, one of the main influential factors is the presence of other ions in the solution. Accordingly, what follows includes a reflection on NaCl in a common salt mixture.
NaCl in a salt mixture—thermodynamic modelling with ECOS/RUNSALT
To illustrate the experimental determination of crystallization cycles in realistic climatic conditions it is important to consider that single salts are rarely present in nature. On average a typical building material subjected to decades of water infiltration will contain a mixture of different ions, such as, Cl−, NO3−, SO42−, CO32−, Na+, K+, Ca2+, Mg2+. The salt content of a deteriorating limestone in the Cathedral of Our Lady in Antwerp is used to illustrate the influence of adding other ions to a binary system containing Na+ and Cl−. Using the ECOS/RUNSALT model NaCl precipitates below the RHeq of 75.3% at 20 °C) and below the RHeq of 75.5% at 10 °C. However, when the system contains other ions the RHeq of NaCl changes (Fig. 5). In the specific mixture presented NaCl will precipitate between the RH range of 65.5 and 62.4% at 20 °C. In the latter, the complexity of the mixture might also allow precipitation of double salts such as darapskite. The RHeq of NaCl in a mixture will typically shift to a lower RH and is often seen in such outputs ≈ 65%. Again, this shows the need to experimentally verify the behavior of salts in a mixture under changing climatic conditions.
Predicting the occurrence of dissolution cycles—meteorological data resolution (spatial and temporal)
From the experimental data described above a threshold approach to analyze meteorological or climate data based on the RHeq of salts can be applied and has become an established method for testing the environmental risk related to salt damage. As mentioned in the introduction, most research uses daily averages of relative humidity, although some research takes advantage of multiple-hour averages. The pragmatic use of these time periods seems to relate to data availability. As pointed out by Camuffo [37], the frequency of observation should be related to the type of problem. In the case of salt phase transitions, this means taking into account the kinetic behavior influenced by a wide range of factors. Results of the salt crystallization tests in unconfined (Fig. 4) and confined media (Table 1) for a single salt NaCl show a kinetic dependency on confinement, crystal size, ambient RH, RH oscillation, crystallinity, etc. This currently hinders an accurate time integration in an indicator based on a threshold approach. Nevertheless, these experiments are valuable to inform risk assessment based on climate data.
-
First, they show that dissolution is mostly slower, hence being the limiting factor on crystallization-dissolution cycling. This is valid for larger RH gradients (90–< 70–90% RH). When solution is stable at 90% RH, realistic time periods range from sub-hourly for unconfined single salt droplets to multiple hours for confined droplets. Hence it is reasonable to say these can realistically occur on a semi-diurnal scale. For smaller RH gradients (90–70–90%), the time of crystallization is longer than the time for dissolution.
-
Second, the phase transition kinetics speeds up with larger RH gradients, i.e. the time for crystallization and dissolution becomes shorter. This means that it should be investigated if RH extremes (minima and maxima) are relevant in any parameterization.
-
Third, as illustrated by the experiments in capillaries, the kinetics depend upon the depth in the capillary and hence the depth where the crystal or solution is located in the pore network of the stone.
Using meteorological data is only relevant for salt transitions near the surface of a stone, as the material itself buffers strong RH fluctuations at depth. When climate parameterization would include short time windows (< 1 h), this effect already plays at depths of approximately 360 µm below the surface in pores of 100 µm diameter (Table 1). As it is impractical to measure RH at a high spatial resolution inside a pore network, hygrothermal models (also called heat-air-moisture models) could provide valuable input on RH gradients below the surface. It may even impact the conditions at the surface, emphasizing the need for microclimatic observations at the surface.
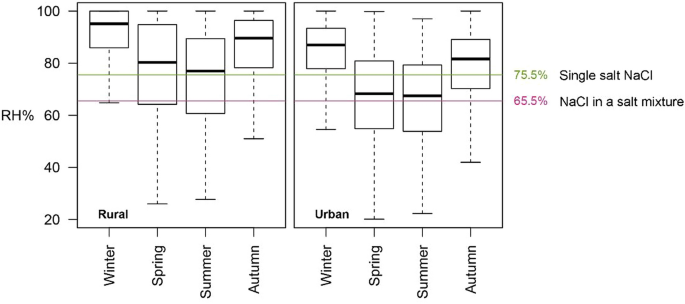
Grossi et al. [29] introduced the idea of salt climatology which implies a regional and seasonal dependency of the environmental risk of salt phase transitions. Additionally, it has been shown for other environmental risks such as freeze–thaw action that subregional phenomena such as the urban heat island [38] and microclimates around buildings, e.g., as the result of orientation [39], have a significant impact. The importance of both seasonality as well as local climate effects is also illustrated in this paper. Figure 6 presents the seasonal RH variations for the urban and rural meteorological data in and around Ghent (Belgium), using high-frequency meteorological observations of an urban environment in Ghent and its rural surroundings (data obtained from Caluwaerts et al. [33]). Grossi et al. [29] evidenced that more NaCl phase transitions are found when the monthly mean relative humidity is close to the 75% RH threshold. They found a sharp increase during spring months stabilizing in summer before declining in autumn, using meteorological data of weather stations in northwestern France. This trend is also observed in the dataset presented in Fig. 6, showing that seasonal averages are much closer to the transition point during spring and summer (for each of the indicator thresholds), suggesting a higher risk in these seasons. Even though extremes in winter and autumn cross the threshold, the averages are well above the threshold of both indicators, with an exception in the urban environment when considering the threshold of a NaCl as a single salt.
The RH distributions (standard boxplots; thick black lines = medians, and box extents showing the upper and lower quartiles (25th and 75th percentiles, respectively) per season in rural (Melle, Belgium, 2016) or urban (central Ghent, Belgium, 2016) environments, demonstrating that the rural/urban difference of expected crystallization is most pronounced in spring and summer since the threshold of 75.5% and 65.5% are crossed
The average RH is generally lower in the urban environment compared to the rural environment. Using a 75.5% threshold, the average RH in the rural environment is closer to the threshold in spring and summer, whilst it is closer for the urban environment in autumn and winter, suggesting a higher risk in spring/summer in the rural environment and higher risk in autumn/winter in the urban environment. Using a 65.5% threshold, the risk is higher in the urban environment throughout the entire year, with all seasonal averages closest to the threshold. This illustrates the limitations of generalizations when studying the actual damage risk under different or predicted environments.
Additionally, there is also a dependency of the results on the averaging time window of measurement. Figure 7 shows the number of NaCl phase transitions using a 75.5 and 65.5% RH threshold, respectively based on RHeq for pure NaCl and NaCl in a mixture, for different time windows of observation and environmental context. For time windows shorter than 10 min, the rural environment has a higher amount of fluctuations throughout all seasons, which is not reflected in the datasets based on averages in Fig. 6. It is likely that high-frequency fluctuations (shown in Fig. 7) are buffered by the urban heat island effect. This is more pronounced in spring and summer, likely relating to short but extreme weather events occurring in those seasons [40]. However, these high-frequency RH fluctuations possibly have less impact on salt crystallization due to slower kinetics of salt dissolution. A time window of 1 min therefore illustrates an unrealistic high number of cycles, easily exceeding 200 cycles and sometimes almost approaching 800 per season, resulting in almost 2500 cycles per year. This is not relevant for salt phase transitions that experimentally don’t occur on this timescale (Fig. 4 and Table 1). This result, in combination with the dissolution time on the magnitude of the change in RH%, will influence the implementation of risk assessment based on climate data. The time interval of change, and therefore the required data resolution and/or averaging, should be based on the magnitude of change in RH% to more accurately reflect realistic salt behavior.
Number of dissolution/crystallization cycles of NaCl per season over one year in a rural (Melle, Belgium, 2016) or urban (central Ghent, Belgium, 2016) environment considering an average time scale between 1 min and 24 h when the RH threshold of 75.5% (representing pure NaCl) or 65.5% (representing NaCl in a mixture) is surpassed (x-axis in log scale)
There is a sharp decrease in number of cycles when enlarging the time window from one to 30 min. The trend between this quantitative approach and the qualitative evaluation of Fig. 6 is in agreement when discarding high-frequency data (< 20 min). When considering a time window of approximately 30 min, nearer to the observed dissolution time of droplets and smaller solution volumes in the capillaries presented herein (Fig. 4 and Table 1), the number of cycles is less than half compared to the maximum (at 1 min) for both urban and rural environments. There is a steady decrease in number of cycles between 30 min and 6 h. On the other hand, it can be observed that the number of cycles taken on a daily average (24 h) is well below the amount of these multiple-hour averages. Daily averages might underestimate the number of cycles if one would assume that phase transitions can occur in the range of a few hours as illustrated by the capillary experiments (Table 1). Whilst it is realistic to reject time windows below 20 min the use of (semi-)hourly data versus (semi-)diurnal has a significant effect on the estimated number of cycles.
The trends observed in Fig. 6 are confirmed by the data of Fig. 7. The overall number of cycles is higher in spring and summer for both environments. Nevertheless, when considering a 75.5% threshold of a single salt, the risk is higher in a rural environment, whereas this is the case for the urban environment when considering a 65.5% threshold for NaCl in a salt mixture (of course, such conclusions are overall depending on the general climate of the area).
Conclusions
The research presented supports the prediction of salt weathering over time to underpin risk assessment. The importance of considering salt kinetics when estimating the number of crystallization cycles in a given environment is influenced by factors, such as the exposed surface area of crystals/solution (unconfined versus confined), the behavior of a single salt versus mixture, the resolution and location of climate measurements. The experimental data show that mostly dissolution processes take longer than crystallization, and this might be the deterministic time step in the use of climate data for risk assessment. Also the gradient of changes in RH, the minimum and maximum within a cycle, strongly affects crystallization/dissolution time and should therefore be taken into account. At low RH gradients, the crystallization time drastically increases, illustrated by the 90–70–90% RH cycle, and here crystallization time is more relevant.
The determination of relevant RH thresholds (RH equilibrium for a single salt or equilibria for a salt mixture) is also a critical factor. For NaCl the calculations show how the RHeq drops when found in a common salt mixture and affects the accuracy of the prediction and reliability of the model. It has also been shown that specific location data is crucial for the assessment and that climate data at low spatial resolution (i.e. one site used to represent an entire urban area) can significantly influence the frequency of cycles. Seasonal buffering demonstrates the importance of the urban context and microclimates, rather than typically using measured meteorological data.
These results illustrate how the use of an indicator based on a single salt does not represent the more common real-world scenario of salts present in a mixture. The application of a simple indicator (threshold, based on a variable ‘averaging window’ of time) demonstrates a significant difference in estimated number of cycles. The findings are fundamental to improve appropriate timescale windows and illustrate a methodology with specific points of interest to quantify salt crystallization cycles in realistic environments as a risk assessment procedure that can be applied to climate data. Of additional interest is the potential to build upon the proposed method by incorporating one or a combination of other important factors and assessment approaches, including salt mixtures, pore and salt filling properties, extended climatic factors, boundary conditions, urban heat island effects, climate projections, and hygrothermal simulation.
Availability of data and materials
All data supporting and presented in this paper is saved on the servers at the Royal Institute for Cultural Heritage, Brussels, Belgium (KIK-IRPA) and backed up online, on request the data can be made available by contacting the corresponding author or the Monuments lab at KIK-IRPA.
Abbreviations
- RHeq :
-
relative humidity equilibrium
- aw :
-
water activity
- sccm:
-
standard cubic centimeters per minute
- μg:
-
microgram
- μm:
-
micrometer
- µl:
-
microliter
- mol kg− 1 :
-
moles per kilogram of solvent (water) (molality)
- tx :
-
time
- dx:
-
distance
- Vx:
-
volume
- C:
-
crystallization
- D:
-
dissolution
- N2 :
-
dinitrogen
- NaCl:
-
sodium chloride
- Cl− :
-
chloride
- NO3 − :
-
nitrate
- SO4 2 − :
-
sulfate
- Na+ :
-
sodium
- K+ :
-
potassium
- Ca2 + :
-
calcium
- Mg2 + :
-
magnesium
References
KNOWMORE network. Heritage stone Monitoring and Remediation: knowledge exchange placement). The Belgian Federal Scientific Policy Office (BELSPO) funded a two-year initiative (2019-2021) focused on fostering collaboration and knowledge exchange between KI [Internet]. Available from: http://www.belspo.be/belspo/fedra/proj.asp?l=en&COD=BL%2F39%2FFWI+26. Accessed 23 Feb 2021
Joint PhD project PREDICT, Phase Transitions of Salts under Changing Climatic Conditions. Funded by the Belgian Federal Scientific Policy Office (Belspo), BRAIN 2.0 [Internet]. Available from: https://predict.kikirpa.be/. Accessed 9 Oct 2020
Goudie A, Viles H. Salt weathering hazards. Geogr J. 1997;165(2):234.
Evans IS. Salt crystallization and rock weathering: a review. Rev Geomorphol Dyn. 1970;19:153–77.
Charola AE. Salts in the deterioration of porous materials: an overview. J Am Inst Conserv. 2000;39(3):327–43.
Doehne E. Salt weathering: a selective review. Geol Soc Spec Publ. 2002;2002(205):51–64.
Doehne E, Price CAA. Stone Conservation: An Overview of Current Research, 2nd edition. Getty Conservation Institute; 2010. 164 p. http://www.getty.edu/conservation/publications/pdf_publications/stoneconservation.pdf. Accessed 1 Oct 2011.
Siegesmund S, Snethlage R. Stone in architecture: properties, durability. 5th ed. Berlin: Springer Science & Business Media; 2014. p. 1–550.
Steiger M. Salts in porous materials: thermodynamics of phase transitions modeling, and preventive conservation thermodynamics of phase equilibria relevant to salt damage of porous materials. Restoration Build Monuments. 2005;11(6):419–30.
Steiger M. Crystal growth in porous materials—II: influence of crystal size on the crystallization pressure. J Cryst Growth. 2005;282(3–4):470–81.
Flatt RJ, Steiger M, Scherer GW. A commented translation of the paper by C.W. Correns and W. Steinborn on crystallization pressure. Environ Geol. 2007;52(2):187–203.
Flatt RJ, Caruso F, Sanchez AMA, Scherer GW. Chemo-mechanics of salt damage in stone. Nat Commun. 2014;5:1–5.
Flatt R, Aly Mohamed N, Caruso F, Derluyn H, Desarnaud J, Lubelli B, et al. Predicting salt damage in practice: a theoretical insight into laboratory tests. RILEM Tech Lett. 2017;2:108–18.
Sawdy A, Heritage A. Evaluating the influence of mixture composition on the kinetics of salt damage in wall paintings using time lapse video imaging with direct data annotation. Environ Geol. 2007;52(2):319–31.
Sawdy A, Heritage A, Pel L. A review of salt transport in porous media, assessment methods and salt reduction treatments. In: Salt weathering on building and stone sculptures, SWBSS proceedings from the first international conference, 22–24 October 2008 Copenhagen: The National museum of Denmark. 2008. p. 1–28.
Derluyn H. Salt transport and crystallization in porous limestone Neutron-X-ray imaging and poromechanical modeling. Diss. ETH No. 20673, 2012. https://doi.org/10.3929/ethz-a-007578301
Desarnaud J, Derluyn H, Molari L, De Miranda S, Cnudde V, Shahidzadeh N. Drying of salt contaminated porous media: Effect of primary and secondary nucleation. J Appl Phys. 2015. https://doi.org/10.1063/1.4930292.
Meldrum F, O’Shaughnessy C. Crystallization in confinement. Adv Mater. 2020;32:2001068.
Lubelli B, Cnudde V, Diaz-Goncalves T, Franzoni E, van Hees RPJ, Ioannou I, et al. Towards a more effective and reliable salt crystallization test for porous building materials: state of the art. Mater Struct. 2018;51(2):55.
Sabbioni C, Brimblecombe P, Cassar M, editors. The atlas of climate change impact on European cultural heritage: scientific analysis and management strategies. London: Anthem Press; 2010.
Steiger M, Charola AE, Sterflinger K. Weathering and deterioration. In: Siegesmund S, Snethlage R, editors. Stone in architecture: properties, durability. Berlin: Springer; 2014.
Price CA, editor. An expert chemical model for determining the environmental conditions needed to prevent salt damage in porous materials. In: European commission research report No 11, protection and conservation of European cultural heritage. London: Archetype Publications; 2000.
Bionda D. RUNSALT - A graphical user interface to the ECOS thermodynamic model for the prediction of the behaviour of salt mixtures under changing climate conditions [Internet]. 2005. Available from: http://science.sdf-eu.org/runsalt/. Accessed 19 Oct 2020
Godts S, Hayen R, De Clercq H. Investigating salt decay of stone materials related to the environment, a case study in the St. James church in Liège, Belgium. Stud Conserv. 2017;62(6):329–42.
Shen Y, Linnow K, Steiger M. Crystallization behavior and damage potential of Na2 SO4—NaCl mixtures in porous building materials. Cryst Growth Des. 2020;20:5974–85.
Lindström N, Talreja T, Linnow K, Stahlbuhk A, Steiger M. Applied geochemistry crystallization behavior of Na 2 SO 4 e MgSO 4 salt mixtures in sandstone and comparison to single salt behavior. Appl Geochem. 2016;69:50–70.
Steiger M, Linnow K, Ehrhardt D, Rohde M. Decomposition reactions of magnesium sulfate hydrates and phase equilibria in the MgSO4-H2O and Na+-Mg2+-Cl–SO42–H2O systems with implications for Mars. Geochim Cosmochim Acta. 2011;75(12):3600–26.
De Clercq H, Jovanović M, Linnow K, Steiger M. Performance of limestones laden with mixed salt solutions of Na2SO4-NaNO3 and Na2SO4-K2SO4. Environ Earth Sci. 2013;69(5):1751–61.
Grossi CM, Brimblecombe P, Menéndez B, Benavente D, Harris I, Déqué M. Climatology of salt transitions and implications for stone weathering. Sci Total Environ. 2011;409(13):2577–85.
Benavente D, Sanchez-Moral S, Fernandez-Cortes A, Cañaveras JC, Elez J, Saiz-Jimenez C. Salt damage and microclimate in the Postumius Tomb, Roman Necropolis of Carmona. Spain Environ Earth Sci. 2011;63(7–8):1529–43.
Menéndez B. Estimation of salt mixture damage on built cultural heritage from environmental conditions using ECOS-RUNSALT model. J Cult Herit. 2017;24:22–30.
Menéndez B. Estimators of the impact of climate change in salt weathering of cultural heritage. Geosciences. 2018;8(11):401.
Caluwaerts S, Hamdi R, Top S, Lauwaet D, Berckmans J, Degrauwe D, et al. The urban climate of Ghent, Belgium: a case study combining a high-accuracy monitoring network with numerical simulations. Urban Clim. 2020;31:100565.
Arfvidsson J. A new algorithm to calculate the isothermal moisture penetration for periodically varying relative humidity at the boundary. Nord J Build Phys. 1999;2:11.
Desarnaud J, Shahidzadeh-Bonn N. Salt crystal purification by deliquescence/crystallization cycling. EPL. 2011;95(4):48002.
Desarnaud J, Derluyn H, Carmeliet J, Bonn D, Shahidzadeh N. Metastability limit for the nucleation of NaCl crystals in confinement. J Phys ChemLett. 2014;5(5):890–5.
Camuffo D. Microclimate for Cultural Heritage [Internet]. 1st ed. Elsevier Science; 1998. Available from: https://www.elsevier.com/books/microclimate-for-cultural-heritage/camuffo/978-0-444-82925-2. Accessed 6 June 2020
Guilbert D, Caluwaerts S, Calle K, Van Den Bossche N, Cnudde V, De Kock T. Impact of the urban heat island on freeze-thaw risk of natural stone in the built environment, a case study in Ghent, Belgium. Sci Total Environ. 2019;677:9–18.
McAllister D, McCabe S, Smith BJ, Srinivasan S, Warke PA. Low temperature conditions in building sandstone: the role of extreme events in temperate environments. Eur J Environ Civ Eng. 2013;17(2):99–112.
Goudenhoofdt E, Delobbe L. Statistical characteristics of convective storms in belgium derived from volumetric weather radar observations. J Appl Meteorol Climatol. 2013;52(4):918–34.
Acknowledgements
This paper is the extended result of a network project KNOWMORE (Heritage stone Monitoring and Remediation: knowledge exchange placements (2019–2021) between the Royal Institute for Cultural Heritage (KIK-IRPA), Ghent University, Oxford University and The Belgian Building Research Institute (BBRI) and an introduction to the continuing BRAIN 2.0 joint PhD project PREDICT, Phase Transitions of Salts under Changing Climatic Conditions. The authors wish to thank Prof. Dr. Heather Viles (University of Oxford) for her valuable input and inspirational discussions during the KNOWMORE project. We also wish to thank Prof. Dr. Steven Caluwaerts (UGent) and the MOCCA project (http://observatory.ugent.be) for climate data. Alexandre Gillon, (Polytech Clermont-Ferrand, Engineering Physics, France) is thanked for his help with the analysis of NaCl crystallization-dissolution carried out as part of his distance learning internship at KIK-IRPA. We acknowledge Alice Vrancken (University of Antwerp, ARCHES), as the results from her Master thesis supported decisions made throughout the experimental and theoretical work.
Funding
This research was funded by the Belgium Science Policy (Belspo) within the framework of BRAIN-be 2.0, Belgian Research Action through Interdisciplinary Networks: project B2/191/P1/PREDICT (Research action B2); joint PhD project PREDICT, Phase Transitions of Salts under Changing Climatic Conditions. The authors further acknowledge the funding for the GenRH humidity generator through “BOF, project UG_2832369580”, “JPI-JHEP project KISADAMA” and “FWO Research Grant 1521815N”.
Author information
Authors and Affiliations
Contributions
SG, SAO, JD and TDK: Conceptualization, Methodology, Data curation, Writing- Original draft preparation, Visualization, Investigation. MS, KW, HDC and VC: Supervision, Validation, Writing- Reviewing and Editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Godts, S., Orr, S.A., Desarnaud, J. et al. NaCl-related weathering of stone: the importance of kinetics and salt mixtures in environmental risk assessment. Herit Sci 9, 44 (2021). https://doi.org/10.1186/s40494-021-00514-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40494-021-00514-3