- Research article
- Open access
- Published:
Micro differential scanning calorimetry and micro hot table method for quantifying deterioration of historical leather
Heritage Science volume 7, Article number: 48 (2019)
Abstract
The aim of this study was to advance the current understanding on the mechanism of deterioration of historical vegetable tanned leathers and establish new criteria for quantifying their deterioration using micro differential scanning calorimetry (micro DSC) and micro hot table (MHT) method. Ten historical leather objects were investigated to this purpose. The calorimetric indices of macromolecular change identified for fibrous collagen provided quantitative results on the bulk material and deconvolution of DSC denaturation peaks revealed the dynamics of deterioration in historical leather. The results brought clear evidence that long-term natural ageing of leather induces the destabilization of chemically modified collagen thereby promoting its partial de-tanning and allowing micro-unfolding of chemically unmodified collagen. As deterioration continues toward the extreme, collagen molecules become highly unstable allowing for gelatinisation and irreversible denaturation. It is thus explained the coexistence of collagen populations with distinct thermal stability in historical leather. They are grouped in three main structural domains, namely “leather-like”, “parchment-like” and “gelatine-like”, whose mass percentages determines leather stability against further natural ageing and deterioration. As a result of the MHT and micro DSC parameters correlation, a more comprehensive set of criteria, including Tf and Tl values, as well ∆C and ∆T intervals’ lengths, was introduced for better interpreting the shrinking activity of collagen in historical vegetable-tanned leathers and open thus the way for the highly sought in situ evaluation of leather artefacts.

Introduction
Historical leathers, in a huge variety of items as footwear and garments, bookbinding, wall tapestry, upholstery, harnesses, armours, storage vessels, household tools, cases, musical instruments, toys, ritual objects, are important parts of our cultural heritage, from remote time to modernity. It is vital therefore that these objects remain well preserved along with all the knowledge involved, from their material aspects and value of use to historical, cultural and artistic values. However, analysis and diagnosis of historical objects is especially challenging owing to the fact they are unique and irreplaceable, which demands that analytical investigation must be non-invasive or, at the very least, employ microsamples. The quality of the information obtained (descriptive, explanatory, quantitative) is a critical factor when selecting the investigating technique. Ensuring the optimum ratio between the invasiveness of investigation and quality of data provided is generally a difficult task, and in the case of leather artefacts the difficulty increases due to their damage non-uniformity which lead to complex composition (old leathers are complex materials constituted of tanned collagen, untanned collagen and gelatinised collagen) and structural heterogeneity.
For rendering the raw animal skin, a more useful and durable material, i.e. non-putrescible under warm and moist conditions, vegetable extracts, fermented materials, fats or aluminium salts have been used since the end of Neolithic [1, 2]. Leather can thus be considered as the first biomaterial ever produced. The term “tanning” (tannage in French) was originally reserved only for the leather production with tannins [3, 4], while the term “tawing” was used to indicate the method of tanning with aluminium salts (alum, a double salt of aluminium and potassium phosphate, occurs naturally in many warm climates, hence it’s early use as a tanning agent). The more versatile vegetable-tannage has continuously progressed to become the most used method to produce leather in Western and Mediterranean Europe until the end of the XIXth century [5, 6], when chromium mineral tanning came into use and gradually replaced vegetable tannins. Tannins derive their name from the Latin word tannum, which means “crushed oak bark,” since in early times oak trees served as a major source of tannin for the leather production. Tannins are a class of complex biomolecules of polyphenolic nature synthesized by a large variety of plants that have the property of combining with proteins, cellulose, gelatin, and pectin to form an insoluble complex [7]. Depending on the main polyphenolic constituent, they are classified in condensed tannins, also named proanthocyanidins, and hydrolysable tannins, which comprise two subclasses, gallotannins and ellagitannins. Condensed tannins are oligomeric or polymeric flavonoids whereas hydrolysable tannins consist of a polymer containing a polyol core (d-glucose is the commonest) multi-esterified with gallic acid (gallotannins) or its oxidized derivative, ellagic acid (ellagitannins) [8]. Today, the terms tanning and tannage are applied for all different processes of leather making which include vegetable tanning with plant polyphenols, mineral tanning with metal salts, oil and aldehyde tannages, synthetic tanning agents and organic tannages based on natural polyphenols or synthetic organic oligomers [9].
The chemical nature of collagen, the main constituent of animal skin, allows it to react with a variety of tanning agents, resulting in skin conversion to leather, a much more resistant material whose appearance and properties enables a wide range of uses. De facto, the purpose of tannage is primarily to increase the hydrothermal stability of native collagen, secondarily to increase its biological inertness, and finally, to improve the utility of the hide’s physical properties. The hydrothermal stability can be measured by observing the temperature at which a thoroughly wetted leather specimen experiences shrinkage, when it is heated in water (or glycerine-water solution when the shrinkage temperature is above 98 °C) at a rate of 2 °C per minute (Standard Test Method for Shrinkage Temperature of Leather, ASTM D6076-18). This temperature is called shrinkage temperature, Ts. The collagen fibres shrinking process occurs as a result of the hydrothermal denaturation of the collagen molecules. Even though the tanning reaction is highly complex on the molecular level, to the extent that there is no clear model of its effect on hydrothermal stability (i.e. what causes the Ts to rise), the value of the shrinkage temperature of leather is commonly used as an indicator of the type of tannage or degree of tannage, or both. As denaturation causes collagen to shrink, which is a convenient gross metric, shrinkage has long been used as a measure of heating-induced damage to collagen and collagenous tissues [10, 11] and more recently it is considered a fine measure of the deterioration of ancient leathers [12, 13]. A micro-destructive method called Micro Hot Table (MHT) method that requires a few micro-fibres only and can be easily used by a conservator was set up by Larsen et al. [14] for measuring the hydrothermal stability and hence damage level of historical leather. This method has widely spread in the conservation laboratories and the knowledge gained so far allows for a partly quantitative assessment of deterioration at the fibre level. The MHT method is thus currently used as a routine method to measure Ts and rank the damage level in ancient collagen-based materials such as leather and parchment [15,16,17,18,19]. It has been improved by incorporating an algorithm called imageMHT that allows the automation of the shrinkage intervals determination, with a significant effect on reducing the analysis time, accuracy increasing and inter-laboratory comparison [20]. Yet, MHT assessment is based only on intensive parameters (i.e. not depending on the sample mass) such as temperature which strongly limits the quantitative nature of damage evaluation. It was shown that the high heterogeneity of composition of historical collagenous materials can lead to the overestimation of the “health” condition of a material if only shrinkage temperature measurement is performed [21]. Besides, we must be aware that leather deterioration generally proceeds from the surface to inner layers and can vary from macroscopic to molecular level. Consequently, a comprehensive and reliable damage assessment requires that surface analysis be complemented with bulk analysis, and descriptive information be correlated with explanatory and quantitative information. It is therefore highly advisable that MHT method be always accompanied by another analysis able to provide complementary information on specific alterations of collagen at a different structural level and thus prevent partial or even improper evaluations [22,23,24].
Differential scanning calorimetry (DSC) has been used as an alternative method to precisely measure the shrinkage temperature of historical leathers and obtain information on bulk leather [16, 18]. Although DSC is a powerful quantitative technique which provides a measure of protein (i.e. collagen) hydrothermal stability and an indication of its long-term stability [25,26,27], no quantitative metrics of heat-induced collagen denaturation such as changes in the enthalpy or DSC peak asymmetry have been used so far for assessing damage in historical leather [28, 29]. Badea et al. [30, 31] reported a quantitative assessment of damage in ancient parchments based on the calorimetric parameters associate to collagen denaturation using micro-samples of only 1–2 mg. We have recently used micro DSC to reveal the accelerated ageing induced-deterioration pattern of collagen in vegetable tanned leather and quantify damage in leather after dehydrothermal treatment and visible light exposure [32].
Based on these considerations, in the present study we used both micro DSC technique and MHT method to characterise the deterioration pattern in historical leather at both fibre (shrinkage process) and fibrillar (denaturation process) levels and find quantitative relationships between denaturation/shrinking and deterioration. To this end, a comparison with the outcomes of previous studies on artificially aged and historical parchment and leather [21, 30, 32,33,34,35] was made. Our results provide clear evidence that long-term natural ageing (i.e. exposure to natural environmental fluctuation of temperature, relative humidity and light as well as to the more recent chemical pollutants, and extensive use/manipulation) of leather destabilises tanned collagen thereby promoting partial de-tanning and allowing micro-unfolding of chemically unmodified collagen, and thus progressively lead to its gelatinisation and irreversible denaturation. A further aim of this work was to compare the results obtained by micro DSC with the results obtained by MHT method a to set up a more comprehensive set of criteria for interpreting the shrinkage activity of collagen in historical vegetable tanned leathers.
Materials and methods
Historical leathers
The investigated leather-made objects owned by some Romanian museums are listed in Table 1. All investigated historical leathers appear heavily damaged with dry, shrunk and brittle looking, frequent cracking and spotting. Micro-samples were taken from each historical object, generally from the most fragile areas, inherently exposed to material loss during normal use (i.e. edge, bookbinding spine, etc.).
New vegetable tanned leathers from calf, sheep and goat hides were used for comparison. They were prepared according to procedures inspired by traditional recipes at the National Research and Development Institute for Textile and Leather, Division Leather and Footwear Research, Bucharest (Romanian patent no.122098/2006: Piele naturala pentru legatorie carte de patrimoniu si procedeu de realizare a acesteia). Mimosa bark (condensed tannin) and chestnut wood (hydrolysable tannin) extracts were selected for tanning because both extracts were widely used in the European post-medieval period (the dating period of the investigated historical samples), and remained even nowadays among the mostly used vegetable tanning agents [36]. Chestnut tannin belongs to the ellagitannins [37], while mimosa is a prorobinetinidin polyphenol [38]. Samples were taken from the butt, where fibres are tightly packed making it the strongest part of the skin/leather.
Micro differential scanning calorimetry
Collagen molecules denaturation is a kinetic process [39] and, as such, it must be treated thermodynamically. Micro DSC measurements were carried out with a high-sensitivity SETARAM Micro-DSC III calorimeter using 850 µl stainless steel (Hastelloy C) sample cells. Measurements were performed in the temperature range (5 to 95) °C at 0.5 K min−1 heating rate. Micro-samples of about 2.0 mg were suspended in 0.5 M acetate buffer (pH = 5.0) directly in the measure cell and left for 2 h at 5 °C to assure fully hydration [40] and thus reproducible calorimetric values since it is known that both denaturation temperature and denaturation enthalpy depend on hydration level. It was reported [18] that leather’s thermal characteristics are stable in the pH range 3 to 5. We have previously found that at 5 °C the values of calorimetric parameters of leather thermal denaturation practically remained unchanged for immersion times longer than 2 h indicating that the fibrils had reached their maximum extent in hydration [32]. Depending on the availability, each micro-sample was divided into few very tiny sub-samples and multiple measurements were performed with fresh samples.
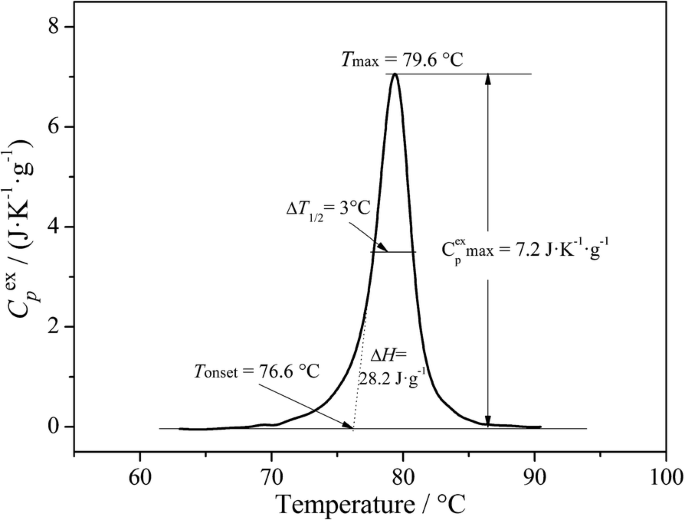
Experimental DSC data acquired with the SETARAM SetSoft2000 software were analysed using PeakFit 4.1 (Jandel Scientific) to obtain the specific heat capacity values of the sample \(C_{p}^{ex} \left( T \right)\) (J K−1 g−1) in the scanned temperature interval and derive the DSC peak parameters featuring collagen fibrils denaturation (Fig. 1). Denaturation temperature, Tmax, was determined as the temperature attained at peak maximum. Temperature span of the transition was reported as peak width at half height, ΔT1/2, and specific denaturation enthalpy, ΔH was calculated as the area under the peak by integrating \(C_{p}^{ex} \left( T \right)\) curve across the denaturation temperature range [26, 32]. DSC multiple peaks of historical leather were deconvoluted using the PeakFit asymmetric Gaussian fit function to improve the fit of the asymmetry in the peaks.
Typical DSC denaturation peak associated with thermal denaturation of fully hydrated collagen fibrils from a new goat leather tanned with mimosa-bark extract. Tonset (°C) is defined by the intersection of a line tangent to the steepest section of the leading edge and the baseline of the thermogram. Tmax (°C) is the temperature of maximum heat flow. Enthalpy of thermal denaturation, ∆H (J/g), is the area under the endothermic peak. ∆T1/2 (°C) is the full width at half maximum of the endothermic peak
It is worth noting that the DSC measurements provide quantitative information on bulk leather whereas the non-invasive investigation techniques are qualitative and mainly relate to the surface of the sample. DSC micro invasiveness could, however, constitute a critical factor for some historical objects/artefacts even if it requires only a minimal amount of sample.
Micro hot table method
The shrinking measurements were carried out using a Linkam LTS120 stage equipped with a temperature controller and Linksys32 temperature control software which enables full PC programming of temperature. The water circulator ensures a temperature stability and accuracy to 0.1 °C and rapid cooling. Shrinkage motion was digitally recorded with a SMZ 745 Nikon stereomicroscope equipped with a Nikon D90 digital camera. This process can be viewed either through the microscope, or on the PC using the camera and image capture and processing software [41]. Of each sub-sample used for micro DSC measurements, few fibres (0.1–0.2 mg) were taken and placed on a microscope slide with a concavity, completely immersed in demineralised and degassed water to ensure thoroughly wetting. Then, the fibre bundles were well separated using fine needles and the microscope slide, covered with a cover glass, placed on the Peltier element of the stage and heated at a rate of 2 °C min−1 in the range (25–95) °C. All measurements were performed three times for each sample and the average values are reported with the standard errors.
Collagen fibres shrinkage activity associated with thermal denaturation is described by a sequence of temperature intervals: no activity—A1–B1–C–B2–A2—complete shrinkage as previously reported [14]. In the first two intervals, A1 and B1, shrinkage discretely occurs in individual fibres and displays higher activity (namely higher amount of shrinkage per unit of time) in B1 interval. Most of the fibre mass shrinks in the main interval ΔC. The starting temperature of this interval is the shrinkage temperature, Ts. Generally, the shrinkage activity levels off through B2 and A2 intervals. Tf is defined as the temperature at which the very first motion is observed while Tl is the temperature of the very last observed motion. The total shrinkage interval is thus calculated as ΔT = Tl − Tf.
Results and discussion
Hydrothermal stability of collagen in leather
Collagen type I (the main constituent of skin) is a hierarchically organised protein characterised by an intimate relationship and connectivity between individual structural levels: molecules, fibrils and fibres [42,43,44]. Collagen fibres are composed of fibrils, which in turn are made up of collagen molecules. The collagen molecule is made of three polypeptide chains, called α-chains, which form a unique triple helix structure which is around 1.4 nm in diameter and 300 nm in length. The primary amino acid sequence of collagen is glycine-proline-X or glycine-X-hydroxyproline, where X can be any of the other 17 amino acids, and every third amino acid is glycine. Hydroxyproline stabilizes the triple helix via a stereoelectronic effect and enables the formation of water-mediated hydrogen bonds that stitch together the folded triple helix. The triple helix configuration is 3 left-handed helices twisted into a right-handed coil. The chemical nature of the side chains attached to the methylene group in the centre of each amino acid residue, i.e. ε-amino group of lysine residues, carboxyl groups of glutamic and aspartic acids, or hydroxyl groups of hydroxylysine and hydroxyproline, ensures intermolecular and interfibrillar cross-linking of collagen with a variety of agents [42] having tanning properties. In addition, the backbone peptide bonds offer different reactions sites that can be exploited during tanning process. The nature and stability of these different chemical bonds produce a significant variation in the properties of the cross-linked product, i.e. appearance, biological, mechanical and thermal properties. One of the most important changes induced by tanning is in fact the increased hydrothermal stability of collagen. This would be due to the increase of packing density of collagen by increasing the density of crosslinking.
According to the latest theory of tanning based on the concept of link-lock [45, 46], all tanning processes are either single- or multi-component. The reaction between collagen and vegetable tannins is a single component reaction termed “linking” because the tanning agent only links to the collagen structure. It confers the same degree of moderate hydrothermal stability (Ts < 85 °C) independently of the tannin type because the bound species merely interfere with the shrinking/denaturation process. For example, the primary link reaction between hydrolysable plant polyphenols and collagen occurs through multiple hydrogen bonding [9]. Similarly, the condensed tannins react with collagen via hydrogen bonds. In addition, they can react via quinoid species, resulting in covalent bonding at the collagen lysine amino group [45]. Multi-component processes additionally involve crosslinking the primary reagent and “locking” the molecules together to create a stable matrix within the collagen structure. This step results in an increase of the shrinkage/denaturation temperature since the collagen-tannin matrix becomes less easily displaced. Further stabilisation of collagen within leather involves the substitution of some of the supramolecular water (i.e. dehydration) and the interaction with some of the remaining water [46].
It is important to underline that, regardless of the tanning mechanism, the denaturation/shrinking process involves the unravelling of the triple helix aided by the disruption of a network of hydrogen-bonded water molecules surrounding the collagen molecule as elegantly explained by Bella et al. [47]. When present as isolated monomers in solution, the triple helix of type I collagen becomes unstable near body temperature, causing molecules to convert into random coils [48]. When packed in a fibril, the proximity of neighbouring molecules restricts the conformational freedom of individual α-chains, substantially increasing the thermal stability of collagen molecules [49, 50]. This mode of stabilisation is known as polymer-in-a-box constraint [51]. Taken together, polymer-in-a-box molecular stabilization combined with the tanning-induced stabilisation of collagen matrix provides adequate explanation for the increased stability of collagen fibrils in leather.
Micro DSC evidences of new leather hydrothermal stability
The parameters of the denaturation process represent a measure of the collagen matrix stability while at the same time provide a measure of the distribution of molecular thermal stabilities [31,32,33]. Figure 1 illustrates the typical sharp endotherm related to the denaturing process of collagen in mimosa-tanned goat leather. This transition of collagen macromolecules from their native triple helical structure into a random coiled structure via unfolding (helix-to-coil transition) is revealed by micro DSC at a defined temperature, Tmax, which has been assumed as denaturation temperature of collagen fibrils according to a statistical process [10]. Besides the position (Tmax) of the DSC peak, its height \((C_{p\text{,max} }^{ex} )\), width (∆T1/2), area (∆H) and symmetry provide valuable information about the denaturation process [52]. In addition, the onset temperature Tonset of the transition, calculated by using the tangent method, is explained by conformational changes occurring within the fibrils before the main denaturation transition Tmax, such as partial shrinkage of the fibrils. This shrinkage phenomenon was also reported by Lin et al. [53], using a second harmonic generation microscopy approach, and by Bozec and Odlyha [54], using localized thermomechanical analysis. We can therefore assume that Tonset and Tmax correspond at macroscopic level to Tf, and Ts, respectively. This is confirmed for a group of 12 samples from 6 new vegetable tanned leathers and 48 artificially aged samples [32] by the good linear correlations obtained when Tmax was plotted versus Ts (R = 0.961) and Tonset versus Tf (R = 0.908) (Fig. 2a, b).
Plots showing a good linear correlation between a Tmax and Ts (correlation coefficient R = 0.961); b Tonset and Tf (correlation coefficient R = 0.908) for a group of 12 samples from 6 new vegetable tanned leathers and 48 artificially aged samples [32]
The experimentally derived enthalpy of denaturation ∆H is the area under the peak and is a measure of the amount of energy required for collagen denaturation, i.e. to disrupt the interactions stabilizing the triple helix. The peak width at half height ΔT1/2 is the full width at half maximum of the endothermic peak and gives a measure of the breadth of the distribution of molecular thermal stabilities.
The calorimetric parameters associated with collagen denaturation in newly obtained vegetable tanned leather are reported in Table 2 together with the corresponding calorimetric parameters of collagen denaturation in parchment. As expected, Tmax in leather clearly depends on the tannin type and show higher values than that of collagen in parchment. Higher values of Tmax (73–80 °C) result from collagen reaction with mimosa condensed tannin than from its reaction with chestnut hydrolysable tannin (66–72 °C), while chemically non-modified collagen in parchments undergoes denaturation at even lower Tmax (55–60 °C). Unlike denaturation temperature, the specific enthalpy (calculated by dividing the total enthalpy by the sample mass) of the new vegetable tanned leathers seems to not significantly depend on the tannin type [32]. However, the denaturation enthalpy of collagen in vegetable tanned leather is much lower than that of parchment [26, 27] and chrome-tanned leather [55, 56]. One reason for this low enthalpic value is the much higher mass percentage of non-collagenous material in vegetable tanned leather compared to parchment and chrome-tanned leather. On the other hand, an influence of the tanning mechanism is expected, since collagen changes its conformation and hydrothermal stability as a result of the specific interactions with tannin. It is only after the complex tannin–collagen breakdown that the denaturation process is controlled by the breaking of hydrogen bonds within the triple helix [44]. In addition, it is worth mentioning that the few enthalpy data from the literature reported by Chahine [18] and Budrugeac et al. [19] for vegetable tanned leather are very scattered and even lower than those measured by us. This can be ascribed, on one hand, to leather high heterogeneity (i.e. non-uniformity in tannage) induced by the fabrication technology and, on the other hand, to the experimental conditions which resulted in various hydration levels. It is known that both Tmax and ∆H depends on the hydration level [40]. So, it is very likely that the very long swelling time (e.g. 12–24 h) used by Chahine and Budrugeac is the cause of altered DSC denaturation parameters [27]. Consequently, long swelling times, are not recommended for historical damaged leather which are very sensitive to excessive moist.
MHT evidences of new leather hydrothermal stability
The parameters associated with collagen fibres shrinking in vegetable tanned leathers are reported in Table 3 together with the corresponding shrinkage parameters of collagen fibres in parchment. It is apparent from these data the distinct shrinkage behaviour of vegetable tanned leather depending on the tannin type and animal species. Mimosa tanned leathers show higher Ts values, as expected, but shorter ΔC and ΔT intervals by comparison with chestnut tanned leathers. This could be attributed to the further covalent interaction of mimosa tannin with collagen compared to chestnut tannin as explained before. Ts values for the investigated new leathers listed in Table 3 well compare with values reported by Larsen et al. [14] as well as with those typically observed for collagen stabilisation by polyphenol reactions (e.g. gallotannin or ellagitannin reaction generally raises Ts to 75–80 °C, whereas flavonoids raise Ts to 80–85 °C). Our slightly higher ΔT intervals may be attributed to the tanning technology.
Deterioration mechanism in historical leather. Quantification of deterioration
Some representative DSC curves associated with hydrothermal denaturation of collagen in historical leather are presented in Fig. 3. The multiple character of the curves 3a, 3b and 3c is well resolved and multiple peaks have been singled out by appropriate deconvolution. Such a thermal behaviour indicates that each denaturation endotherm contains a distribution of collagen molecules with distinct thermal stabilities. Considering that the thermal stabilisation of leather is due to the linking reaction between elements of collagen structure and tannin molecules [46], one can infer that on ageing this linking capability gradually decreases until total de-tanning when leather thermal stability equals that of chemically unmodified collagen in parchment. Further deterioration results in collagen gelatinisation and irreversible denaturation, i.e. the conversion of triple helix into random coils. We can therefore assume that the ageing/deterioration mechanism of leather includes the following key-steps: thermal destabilisation of collagen–tannin complex (chemically modified collagen), de-tanning, thermal destabilisation of chemically unmodified collagen, gelatinisation and denaturation. This assumption is based on our findings concerning the behaviour of both artificially aged leather [32] and parchment [27]. First, thermally destabilised intermediate states of chemically modified collagen are formed through a progressive impairment of collagen–tannin binding. This allows conformational changes and progressive de-tanning and is expressed by the lowering of denaturation temperature. When the breakdown of the collagen–tannin bonding exceeds a hypothetical critical level, full de-tanning occurs. Then, leather exhibits a parchment-like hydrothermal behaviour as we previously reported [32, 57]. Thermally destabilised intermediate states of chemically unmodified collagen are formed through peptide bonds cleavage without disorganising the triple helical structure—this is expressed by further lowering of denaturation temperature [32]. Once enough covalent bonds have been cleaved beyond a hypothetical critical level, shortened peptide segments occur, which contain less than a critical number of hydrogen bonds needed to maintain the coiled-coil, helical structure. As deterioration continues toward the extreme, the stability of some local areas drops below a lower limit between neighbouring crosslinks. At this point, the collagen molecules become highly unstable allowing for gelatinisation and irreversible denaturation. Once the leather structure contains both chemically modified and unmodified collagen, all the steps may occur simultaneously and severely deteriorated collagen progressively accumulates in the leather material. For example, in Fig. 3b we can observe the coexistence of multiple collagen populations: chemically modified collagen with distinct linking capability showing Tmax at 84.4 °C and 69 °C, chemically unmodified collagen showing Tmax at 52.2 °C and gelatinised collagen having Tmax at 42.8 °C. Based on these findings we can characterise the pattern of deterioration of historical leather by simply dividing the temperature range of thermal transitions of collagen in three intervals corresponding to the following structural domains:
a–c Deconvolution of DSC denaturation multi-component peaks for some of the investigated historical leather objects illustrating the distribution of collagen populations with distinct thermal stability and their allocation to the leather-like (L), parchment-like (P) and gelatine-like (G) intervals (see text). d Denaturation endotherms characterised by a main peak indicating the prevalence of one collagen population
-
“Leather-like” (L) domain (65 °C < T < 85 °C), where chemically modified collagen shows denaturation;
-
“Parchment-like” (P) domain (45 °C < T < 65 °C), where chemically unmodified collagen (fully de-tanned) shows denaturation;
-
“Gelatine-like” (G) domain (T ≤ 45 °C), where gelatinised collagen shows thermal transition.
The multiple thermal transitions of the various collagen populations identified by deconvolution can be thus allocated to one of these domains depending on the Tmax value of each DSC peak component. This grouping enables us to also quantify the chemically modified collagen (tanned collagen), chemically unmodified collagen (fully de-tanned collagen) and gelatinised collagen within historical leather based on the percentage of enthalpy of each population (Table 4). It is of outmost importance for conservators to get this information because gelatine-like population may spontaneously denature at room temperature when in the presence of enough moisture content, while leather-like and parchment-like collagen populations are stable and can withstand seasonal temperature and humidity excursions.
Additional information on deterioration comes from the half-width of the overall DSC peak, ∆T1/2 listed in Table 4, too. It is apparent from these data and from Fig. 3a–d that the shape of the overall DSC peak of historical leathers reflects their structural heterogeneity. Very large ∆T1/2 values indicate a distribution of collagen populations in all structural domains whereas very low ∆T1/2 values indicate one major collagen population belonging to a specific structural domain.
It is interesting to note that overall specific enthalpy of collagen denaturation in historical leathers show both lower and higher values than that we measured for collagen in the new vegetable tanned leathers (Table 1). First, it should be stressed that modern tanning technologies use commercial tannins and numerous chemical auxiliaries that greatly accelerate the tanning process, thus reducing the traditional tanning time from 1 to 2 years to 2 to 3 weeks or even a few days. For this reason, there are still no valid references for historical vegetable tanned leathers and hence neither for their calorimetric indices such as enthalpy and temperature of denaturation. The lower ∆H values showed by the historical leathers can undoubtedly be attributed to the collagen content decrease over time as a result of its irreversible denaturation induced by deterioration. In fact, severely deteriorated collagen that does not contribute to the denaturational change in enthalpy accumulates in the leather over time, resulting in a decrease of the overall denaturation enthalpy. Although the higher ∆H values showed by the historical leathers may seem surprisingly at first sight, it is very plausible that the initial enthalpy of leather before the damage occurred was higher than that currently measured by us for the modern vegetable tanned leather. Secondly, the mass loss of the non-collagenous components over time can contribute to an increase of the collagen mass percentage in historical leather. The non-collagenous mass loss may be due to moisture loss, material loss as a result of fungal/microbial growth, powdering phenomena linked to the relatively large tannin and pseudo-tannin molecules which fill the fibre network without binding to collagen as well as to washing out of hydrolysable tannin when leather is exposed to rain, groundwater or surface water. Consequently, the overall ∆H value cannot be used to quantify deterioration of historical leathers as we reported for historical parchments [21, 27, 30, 31]. Nonetheless, the DSC peak deconvolution and collagen populations’ quantification enables us grouping historical leathers in four categories (Table 4):
-
Leather with “leather-like” prevalent behaviour characterised by an enthalpy contribution of the chemically modified collagen populations higher than 50% (e.g. BU-3 and BU-8). Such historical leathers are the most stable on long term.
-
Leather with “parchment-like” prevalent behaviour, with an enthalpy contribution of chemically unmodified collagen populations higher than 50% (e.g. BU-2, BU-5, BU-6, BU-9 and BU-10). These leathers are moderately stable on long term.
-
Leather with “gelatine-like” prevalent behaviour, with an enthalpy contribution of gelatinised collagen populations higher than 50% (e.g. BU-4 and BU-7). Leathers in this group are the least stable and susceptible to irreversible damage.
-
Leather with “mixed” behaviour, for which none of the collagen population contribute more than 50% of the total enthalpy (e.g. BU-1). The long-term stability of these leathers depends on the balance of “leather-like” and “gelatine-like” populations.
Correlation of DSC and MHT parameters for historical leathers
Our data do not confirm the strong linear correlation between Tf and Ts reported by Budrugeac et al. [16]. However, we found a very good correlation between Tmax and Ts values of historical leathers (Fig. 4), as in case of the new and artificially aged leathers (Fig. 2a). This behaviour is not so unexpected since both Tmax and Ts define the same process at various organisation levels i.e. denaturation of collagen fibrils and shrinking of collagen fibres of the major (most abundant) collagen population (Table 4). We can therefore confirm the suitability of using Ts to classify the collagen major population into one of the three groups defined earlier: “leather-like”, “parchment-like” and “gelatine-like”. It should be however considered that the general behaviour of a historical leather is not always determined by its majority population. For example, BU-1 shows a “parchment-like” type majority collagen population (Tmax = 47.5 °C and Ts = 50 °C) while its behaviour is classified as “mixed” since none of the collagen population contribute more than 50% of the total enthalpy. The fact that not always the majority population identified by MHT analysis is responsible for the general behaviour of the historical leather represents one of the limitations of this method.
From the shrinking activity illustrated in Fig. 5 it appears clearly that the investigated historical leathers can be grouped in three categories. The first group (BU-1, BU-2, BU-3 and BU-10) is characterised by lower Tf, Ts and Tl values and larger ∆T intervals compared to the new leathers (Table 3), while ∆C intervals are slightly narrower. This behaviour can be attributed to a high heterogeneity in the structural domains with distinct thermal stabilities and clearly indicates the simultaneous presence of “leather-like” and “parchment-like” populations. When Tf, Ts show very low values, the “gelatine-like” population is also present. In the absence of micro DSC bulk analysis, such leather should be classified as having an “mixed” behaviour and its stability be evaluated according to its Ts value.
The second group (BU-5, BU-6, BU-8 and BU-9) also display low Tf, Ts and Tl values but ∆T and ∆C intervals are similar to those of new leathers. From Table 4 it comes out that these samples are mainly composed of a homogeneous collagen population and we can call them “homogeneous” leathers. For example, BU-5, BU-6 and BU-9 have “homogeneous parchment-like” behaviour, whereas BU-8 has a “homogeneous leather-like” behaviour. Low Tf, Ts and Tl values accompanied by ΔT and ΔC intervals like those of new leathers can therefore be used to identify the “homogeneous” historical leathers, while their behaviour (stability) is indicated by the Ts value.
The third group (BU-4 and BU-7) display the lowest values for Tf, Ts and Tl and ∆C. This latter group is represented by the two samples showing a prevalent “gelatine-like” behaviour (Table 4), with an enthalpy contribution of the gelatinised collagen population of 78% and 66%, respectively. We can thus state that detecting very low values for Tf, Ts, Tl and ΔC is a strong indication of “gelatine-like” behaviour and hence of an advanced state of deterioration.
By comparing the results obtained using micro DSC and MHT method clearly result that the most stable “leather-like” populations (i.e. those displaying Tmax > 80 °C) generally showed no shrinkage motion. This can be partly ascribed to the low or very low content of such populations (Table 4) (that enhances the limitations of surface sampling) and partly to the structural features of chemically modified (tanned) collagen fibres very tightly packed in compact and hard fibre bundles that prevents a good separation before measuring their shrinking activity. These drawbacks are overcome by the micro DSC technique which provides a bulk assessment and, due to its high sensitivity, ensures the detection of all collagen populations.
In conclusion, the capability of Ts to grade deterioration [13] in historical leather appears rather limited. When either micro DSC analysis is not available or sampling of 1–2 mg of sample is not allowed, it is necessary to also consider Ts, Tf, Tl, ∆C and ∆T variation for a more reliable assessment of deterioration.
Conclusions
The micro DSC analysis provided quantitative results on the bulk material and unambiguously pictured the deterioration state of historical leather through several calorimetric indices of macromolecular change identified for fibrous collagen, including (1) the temperature at the endothermic peak maximum (Tmax), (2) the specific enthalpy of denaturation (∆H), (3) heterogeneity in the structural domains with distinct thermal stabilities (∆T1/2). Separation through peak deconvolution of the endothermic peaks associated with these structural domains confirms that historical leathers are complex blends of chemically modified, chemically unmodified and gelatinised collagen having distinct hydrothermal stabilities. Variation of the calorimetric indices and deconvolution of the overall DSC denaturation peak facilitated the interpretation of the changes induced by the natural ageing and identification of the key-steps of deterioration: thermal destabilisation of chemically modified collagen, de-tanning, thermal destabilisation of chemically unmodified collagen, gelatinisation and irreversible denaturation.
The classification of the various collagen populations depending on their Tmax in three main structural domains (i.e. “leather-like”, “parchment-like” and “gelatine-like”) and their quantification based on the enthalpy percent contribution to the overall denaturation enthalpy allowed for a deeper understanding of deterioration steps (dynamics) in ten historical leathers.
All this proves the potential of micro DSC technique to provide a greater practical understanding of deterioration in collagenous materials and characterise the ability of historical leather to respond to environment and withstand further deterioration. Studies of established and experimental treatments and preventive conservation practices would benefit from this method of quantifying deterioration based on the collagen population’s mass percentage distribution.
Besides, by correlating the MHT and micro DSC parameters, the suitability of using Ts to classify the collagen major population into one of the three structural domains was confirmed. In addition, a more comprehensive set of criteria, including Tf and Tl values, as well ∆C and ∆T intervals’ lengths’, was introduced to characterize the heterogeneity in the structural domains with distinct thermal stabilities. This is the first time that an in-depth correlation of micro DSC and MHT results was performed allowing for better interpreting the shrinking behaviour of collagen in historical vegetable tanned leathers. Our results substantiate MHT as a reliable method for in situ testing and evaluation of deterioration of leather artefacts.
Availability of data and materials
Not applicable.
Abbreviations
- DSC:
-
differential scanning calorimetry
- MHT:
-
micro hot table
- INCDTP-ICPI:
-
National Research and Development Institute for Textile and Leather, Division Leather and Footwear Research Institute
References
Forbes RJ. Leather in antiquity. Studies in ancient technology, vol. 5. 1st ed. Leiden: Brill Publishers; 1957. p. 1–77.
Haslam E. Vegetable tannage: where do the tannins go? J Soc Leather Technol Chem. 1997;81:45–51.
Hassan RRA. A preliminary study on using line seed oil emulsion in dressing archaeological leather. J Cult Herit. 2016;21:786–95.
Hassan RRA, Ali MF, Fahmy AA. Use of SEM, FTIR and amino acid analysis methods to assess the damage of some historical leather bindings from the XIXth century, stored in National Archive, Cairo. Int J Conserv Sci. 2018;9(1):127–36.
Leather Thomson R. In: May E, Jones M, editors. Conservation science: heritage materials. Cambridge: The Royal Society of Chemistry; 2006. p. 67.
Falcão L, Araújo MEM. Tannins characterisation in new and historic vegetable tanned leathers fibres by spot tests. J Cult Herit. 2011;12(2):149–56.
Bate-Smith EC, Swain T. Flavonoid compounds. In: Mason HS, Florkin AM, editors. Comparative biochemistry. New York: Academic Press; 1962. p. 755–809.
Khanbabaee K, van Ree T. Tannins: classification and definition. Nat Prod Rep. 2001;18:641–9.
Covington AD. Modern tanning chemistry. Chem Soc Rev. 1997;26:111–26.
Weir CE. Rate of shrinkage of tendon collagen—heat, entropy, and free energy of activation of the shrinkage of untreated tendon. Effect of acid, salt, pickle, and tannage on the activation of tendon collagen. J Am Leather Chem Assoc. 1949;44:108–40.
Pankhurst K. “Incipient shrinkage” of collagen and gelatin. Nature. 1947;159:538.
Larsen R. Experiments and observations in the study of environmental impact on historical vegetable tanned leathers. Thermochim Acta. 2000;365:85–99.
Larsen R, Poulsen Sommer DV, Mühlen Axelsson K. Scientific approach in conservation and restoration of leather and parchments objects in archives and libraries. In: Engel P, editor. New approaches to book and paper conservation-restoration. Wien: Verlag Berger; 2011. p. 239–58.
Larsen R, Vest M, Nielsen K. Determination of hydrothermal stability (shrinkage temperature) of historical leathers by Micro Hot Table technique. J Soc Leather Technol Chem. 1993;77:151–6.
Carsote C, Kövari L, Albu C, Hadîmbu E, Badea E, Miu L, Dumitrescu G. Bindings of rare books from the collections of the Romanian Academy Library—a multidisciplinary study. Leather Footwear J. 2018;18(4):307–20.
Budrugeac P, Carşote C, Miu L. Application of thermal analysis methods for damage assessment of leather in an old military coat belonging to the History Museum of Braşov-Romania. J Therm Anal Calorim. 2017;127(1):765–72.
Mühlen Axelsson K, Larsen R, Poulsen Sommer DV, Melin R. Degradation of collagen in parchment under the influence of heat-induced oxidation: preliminary study of changes at macroscopic, microscopic, and molecular levels. Stud Conserv. 2016;61(1):46–57.
Chahine C. Changes in hydrothermal stability of leather and parchment with deterioration: a DSC study. Thermochim Acta. 2000;365:101–10.
Budrugeac P, Miu L. The suitability of DSC method for damage assessment and certification of historical leathers and parchments. J Cult Herit. 2008;9:146–53.
Miu OA, Badea E, Carsote C, Ciobanu S. Automatic detection of collagen fibres shrinkage activity using Σ-Δ filtering. In: Proceedings Book of the 5th international conference on advanced materials and systems (ICAMS 2014). Bucharest: Certex; 2014. p. 539–42.
Badea E, Poulsen Sommer DV, Mühlen Axelsson K, Larsen R, Kurysheva A, Miu L, Della Gatta G. Damage ranking in historical parchments: from microscopic study of fibres structure to collagen denaturation assessment by micro DSC. e-Preserv Sci. 2012;9:97–109.
Badea E, Şendrea C, Carşote C, Adams A, Blümich B, Iovu H. Unilateral NMR and thermal microscopy studies of vegetable tanned leather exposed to dehydrothermal treatment and light irradiation. Microchem J. 2016;129:158–65.
Sendrea C, Carșote C, Radu M, Badea E, Miu L. The effect of gamma irradiation on shrinkage activity of collagen in vegetable tanned leather. Rev Chim. 2017;68(7):1535–8.
Sebestyén Z, Jakab E, Badea E, Barta-Rajnai E, Şendrea C, Czégény ZS. Thermal degradation study of vegetable tannins and vegetable tanned leathers. J Anal Appl Pyrol. 2019;138:178–87.
Gill P, Moghadam TT, Ranjbar B. Differential scanning calorimetry techniques: applications in biology and nanoscience. J Biomol Tech. 2010;21(4):167–93.
Badea E, Della Gatta G, Usacheva T. Effects of temperature and relative humidity on fibrillar collagen within parchment: a micro Differential Scanning Calorimetry (micro DSC) study. Polym Degrad Stab. 2011;97:346–53.
Badea E, Usacheva T, Della Gatta G. The use of differential scanning calorimetry to characterise collagen deterioration in parchment. Rossiiskii Khimicheskii Zhurnal - Zhurnal Rossiiskogo Khimicheskogo Obshchestva im. D.I. Mendeleeva (Russian Chem J). 2015;59(1):28–41.
Miles CA. Kinetics of collagen denaturation in mammalian lens capsules studied by differential scanning calorimetry. Int J Biol Macromol. 1993;15:265–71.
Samouillan V, Delaunay F, Dandurand J, Merbah N, Gardou JP, Yousfi M, Gandaglia A, Spina M, Lacabanne C. Use of thermal techniques for the characterization and selection of natural biomaterials. J Funct Biomater. 2011;2:230–48.
Della Gatta G, Badea E, Ceccarelli R, Usacheva T, Mašić A. Assessment of damage in old parchment by DSC and SEM. J Therm Anal Calorim. 2005;82:637–49.
Badea E, Della Gatta G, Budrugeac P. Characterisation and evaluation of the environmental impact on historical parchments by DSC. J Therm Anal Calorim. 2011;104(2):495–506.
Carşote C, Badea E, Miu L, Della Gatta G. Study of the effect of tannins and animal species on the thermal stability of vegetable leather by differential scanning calorimetry. J Therm Anal Calorim. 2016;124(3):1255–66.
Badea E, Miu L, Budrugeac P, Giurginca M, Mašić A, Badea N, Della Gatta G. Study of deterioration of historical parchments by various thermal analysis techniques, complemented by SEM, FTIR, UV-Vis-NIR and unilateral NMR investigations. J Therm Anal Calorim. 2008;91:17–27.
Budrugeac P, Badea E, Della Gatta G, Miu L, Comănescu A. DSC study of deterioration caused by environmental chemical pollutants to parchment, a collagen based material. Thermochim Acta. 2010;500:51–62.
Carşote C, Budrugeac P, Decheva R, Haralampiev NS, Miu L, Badea E. Characterization of a byzantine manuscript by infrared spectroscopy and thermal analysis. Rev Rou Chim. 2014;59:429–36.
Falcão L, Araújo MEM. Vegetable tannins used in the manufacture of historic leathers. Molecules. 2018;23(5):1081.
Tang HR, Hancock RA, Covington AD. Study on the composition and structure of commercial chestnut tanning agent. Basic Life Sci. 1992;59:221–43.
Pasch H, Pizzi A, Rode K. MALDI-TOF mass spectrometry of polyflavonoid tannins. Polymer. 2001;42(18):7531–9.
Miles CA, Burjanadze TV, Bailey AJ. The kinetics of the thermal denaturation of collagen in unrestrained rat tail tendon determined by differential scanning calorimetry. J Mol Biol. 1995;245(4):437–46.
Luescher M, Ruegg M, Schindler P. Effect of hydration upon the thermal stability of tropocollagen and its dependence on the presence of neutral salts. Biopolymer. 1974;13:2489–503.
Miu OA, Ciobanu S, Badea E. Automatic method for damage assessment of historical and archeological leather and parchment. Patent application A/001253-05.07.2014.
Hulmes DJ. Building collagen molecules, fibrils and suprafibrillar structures. J Struct Biol. 2002;137(2):2–10.
Buehler MJ. Nature designs tough collagen: explaining the nanostructures of collagen fibrils. Proc Natl Acad Sci USA. 2006;3(33):12285–90.
Wess TJ. Collagen fibrillas structures and hierarchies. In: Fratzl P, editor. Collagen: structure and mechanics. New York: Springer; 2008. p. 49.
Covington AD. The mechanism of chrome tanning. Glob J Inorg Chem. 2010;1(2):119–31.
Covington A, Song L, Suparno O, Koon H, Collins MJ. Link-lock: an explanation of the chemical stabilisation of collagen. J Soc Leather Technol Chem. 2008;92:1–7.
Bella J, Brodsky B, Berman HM. Hydration structure of collagen. Structure. 1995;9:893–906.
Leikina E, Mertts MV, Kuznetsova N, Leikin S. Type I collagen is thermally unstable at body temperature. Proc Natl Acad Sci USA. 2002;99:1314–8.
George C Na. Monomer and oligomer of type I collagen: molecular properties and fibril assembly. Biochemistry. 1989;28:7161–7.
Tiktopulo EI, Kajava AV. Denaturation of type I collagen fibrils is an endothermic process accompanied by a noticeable change in the partial heat capacity. Biochemistry. 1998;37:8147–52.
Miles CA, Ghelashvili M. Polymer-in-a-box mechanism for the thermal stabilization of collagen molecules in fibers. Biophys J. 1999;76:3243–52.
Miles CA, Bailey AJ. Thermal denaturation of collagen revisited. Proc Indian Acad Sci (Chem. ScL). 1999;111(1):71–80.
Lin SJ, Lo W, Dong CY. Prediction of heat-induced collagen shrinkage by use of second harmonic generation microscopy. J Biomed Opt. 2006;11(3):34020.
Bozec L, Odlyha M. Thermal denaturation studies of collagen by microthermal analysis and atomic force microscopy. Biophys J. 2011;101(1):228–36.
Cucos A, Gaidau C, Badea E, Miu L. Influence of glycerin on denaturation temperature of chrome- and vegetable-tanned leather. Rev Roum Chim. 2015;60(11–12):1093–6.
Yao Q, Wang Y, Chen H, Huang H, Liu B. Mechanism of high chrome uptake of tanning pickled pelt by carboxyl-terminated hyper-branched polymer combination chrome tanning. Chem Select. 2019;4(2):670–80.
Sebestyén Z, Czégény Z, Badea E, Carsote C, Sendrea C, Barta-Rajnai E, János B, Miu L, Jakab E. Thermal characterization of new, artificially aged and historical leather and parchment. J Anal Appl Pyrol. 2015;115:419–27.
Acknowledgements
We thank Prof. Giuseppe Della Gatta for encouragement, discussions, and allowing us to obtain early calorimetric data in his laboratory. Lucretia Miu (INCDTP-ICPI) generously provided the new leathers and parchments. The authors would also like to thank the National Military Museum “Ferdinand I”, the National Museum “Cotroceni”, the National Museum of Romanian History, and the History and Art Museum of Bucharest City for providing the historical samples.
Funding
This work was supported by Grant of the Romanian National Authority for Scientific Research and Innovation, CCCDI-UEFISCDI, project number 56/2017, within PNCDI III.
Author information
Authors and Affiliations
Contributions
Both authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Carsote, C., Badea, E. Micro differential scanning calorimetry and micro hot table method for quantifying deterioration of historical leather. Herit Sci 7, 48 (2019). https://doi.org/10.1186/s40494-019-0292-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40494-019-0292-8